Frequently Asked Questions - Herbal Medicines Compendium
The U.S. Pharmacopeial Convention (USP) is an independent, nonprofit organization that establishes standards that help ensure the quality and benefit of medicines and foods. Since its founding in 1820, USP has published standards for medicines in its flagship compendium, the United States Pharmacopeia (USP). Building on nearly two centuries of standards-setting expertise, USP now publishes additional compendia including the National Formulary (NF), Dietary Supplements Compendium (DSC), Food Chemicals Codex (FCC), USP on Compounding, Medicines Compendium (MC), and, most recently, the new Herbal Medicines Compendium (HMC).
HMC is a freely available, online resource that provides standards for herbal ingredients used in herbal medicines. Standards are expressed primarily in monographs. A monograph contains general information including the definition of the herbal ingredient relative to the monograph title and then follows with a specification. The specification contains tests for critical quality attributes of the herbal ingredient and includes procedures and acceptance criteria. HMC employs validated analytical procedures for the tests of its monographs, using state-of-the-art techniques and allied reference materials. Additional analytical methods and approaches may be referenced in general chapters, which also are available online.
USP believes that public standards are critically important to help ensure the quality of all medicines, including herbal medicines. Through the new HMC, USP now has a forum for advancing standards for herbal ingredients used in traditional medicines worldwide. These ingredients might appear in the United States Pharmacopeia or in an associated compendium, the Dietary Supplements Compendium, but would do so as dietary supplements legally marketed in the United States. HMC will not contain standards for ingredients of animal origin, synthetic chemicals, or biotechnology-derived medicines.
How are standards developed for the Herbal Medicines Compendium?
HMC monographs are developed through a public standards-setting process. A monograph requires information and materials to support its procedures, which form a specification for the herbal ingredient. This information is carefully reviewed by USP’s volunteer Council of Experts, which follows strict conflict of interest and confidential requirements. HMC monographs also move through a public comment process. From research and development to final authorization by the Council of Experts, HMC monographs move through the following stages:
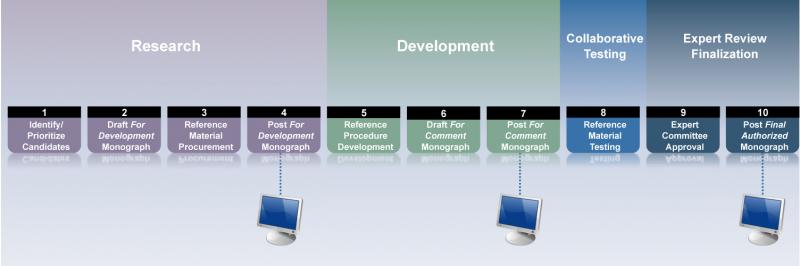
Proposed monographs on the HMC website are initially posted as “For Development,” which indicates more information is needed before they can advance to the next stage. This information is developed by USP staff, working with sponsors and in USP’s facilities. Information involves both research and development as well as collaborative testing of candidate reference materials. Once all the information is complete, monographs are posted as “For Comment.” Public comment at both stages is welcomed. HMC monographs become “Final Authorized’ through decisions of the Council of Experts. Data in support of allied reference materials is also reviewed and, if suitable, approved by the Council of Experts.
The USP Council of Experts has a specific Expert Committee—the USP Dietary Supplements and Herbal Medicines Expert Committee—to finalize HMC monographs and associated reference materials. The Expert Committee comprises volunteer scientists, academicians, and other professionals from around the world elected on the basis of their knowledge and expertise. USP has also established regional Expert Panels that provide scientific support and recommendations on HMC standards to the Expert Committee.
HMC monographs are freely available to any interested party including ingredient manufacturers, herbal product manufacturers, regulatory agencies, and other stakeholders. When coupled with sound registration processes and manufactured according to suitable Good Manufacturing Practices, standards in HMC can become an important part of the safety net that helps ensure access to good quality herbal medicines. HMC standards may be adopted or adapted into a national pharmacopoeia or other related compendium. HMC standards may be used without charge or prior permission from USP. Herbal ingredients eligible for HMC standards include those that have been approved by a national authority for use in herbal medicines or are included in a national pharmacopoeia.
Public participation in the HMC process is critical to the development of USP’s herbal medicine standards. Stakeholders may participate by reviewing and providing input on both “For Development” or “For Comment” HMC monographs. Stakeholders also may provide information and candidate material for new HMC monographs and revisions to "Final Authorized" monographs. For further information on these opportunities, contact Maged Sharaf, Ph.D., Director, Herbal Medicines Compendium, at mhs@usp.org or +1 (301) 816-8318.
HMC standards are not mandatory. Rather, HMC standards are intended as a resource that can be used voluntarily by any party. However, if an HMC monograph or other related standard is adopted for use by an interested stakeholder, it may become subject to enforcement depending on applicable national or other requirements.
Inclusion of a monograph for a particular herbal article in HMC should not be interpreted as an endorsement by USP of its status as a traditional medicine. An article may be approved under different designations in different countries. USP neither supports nor opposes country-specific regulations on how an herbal article is to be regulated or used.
HMC standards represent the quality of herbal articles. Judgments about the safety and efficacy of herbal articles are outside the scope of USP’s decision-making.
An herbal article is considered to be in conformance with an HMC monograph when (1) the identity conforms to that provided in the HMC monograph; (2) the herbal article complies with the specifications in HMC; and (3) the herbal article is analyzed using USP Reference Standards specified in the HMC monograph. These Reference Standards may be considered authorized global primary standards. USP does not endorse and does not review data to ensure the suitability of secondary standards.
A user may label an herbal article “Herbal Medicines Compendium” or “HMC” if the article demonstrates conformance to HMC monograph requirements. If there are multiple procedures in a monograph, then the certificate of analysis or equivalent document must indicate the procedure used. The designation “Herbal Medicines Compendium” or “HMC” does not constitute an endorsement by USP and/or represent assurance by USP that the herbal article is known to comply with all applicable requirements. When the designation “Herbal Medicines Compendium” or “HMC” is used on the label of an article to indicate conformance with HMC standards, the designation shall appear in conjunction with the name of the article. The designation is not to be enclosed in any symbol such as a circle or a square. An article may only be designated “Herbal Medicines Compendium” or “HMC” with regard to the current applicable version of the HMC standard or a directly adopted version; articles may not be designated “Herbal Medicines Compendium” or “HMC” with regard to standards adapted from HMC.
While any herbal article eligible for inclusion in HMC can be developed into a monograph, USP may give priority to articles based on factors including (1) extent of use by the public; (2) interest to multiple countries or regions; (3) interest by a regulatory agency; (4) well-established chemical characteristics through modern separation, spectroscopic, and other analytical techniques; (5) established pharmacologically active component(s) or marker compounds for establishing identity, composition, quality, and purity, and for stability studies; and (6) extent of recognition by multiple national compendia through monographs. These factors take into account public health considerations (e.g., potential patient exposure) as well as practical considerations (e.g., feasibility of monograph development based on available information).
Yes. HMC standards may be adopted or adapted into other pharmacopoeias, including the United States Pharmacopeia and the Dietary Supplements Compendium.
Monographs for herbal articles are included in HMC because of their use in herbal medicines, whereas botanical (herbal) dietary ingredients are included in the United States Pharmacopeia and the Dietary Supplements Compendium because of their use in dietary supplements legally marketed in the United States. These standards may have considerable overlap, but also may differ for reasons that relate to their intended use.
HMC is freely available at hmc.usp.org and through www.usp.org (via the “Around the World” section). Reference materials for HMC may be purchased via the USP Catalog and through the USP iStore (http://www.usp.org/products).
