How are standards developed for the Herbal Medicines Compendium?
How are standards developed for the Herbal Medicines Compendium?
HMC monographs are developed through a public standards-setting process. A monograph requires information and materials to support its procedures, which form a specification for the herbal ingredient. This information is carefully reviewed by USP’s volunteer Council of Experts, which follows strict conflict of interest and confidential requirements. HMC monographs also move through a public comment process. From research and development to final authorization by the Council of Experts, HMC monographs move through the following stages:
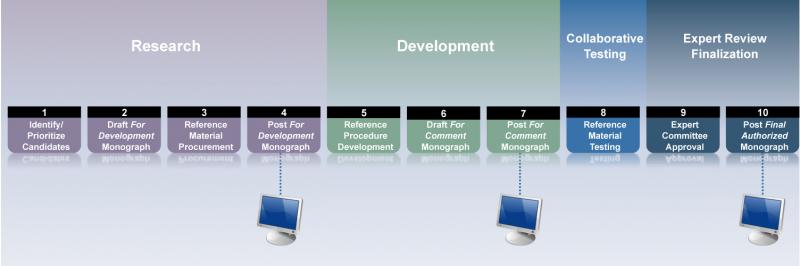
Proposed monographs on the HMC website are initially posted as “For Development,” which indicates more information is needed before they can advance to the next stage. This information is developed by USP staff, working with sponsors and in USP’s facilities. Information involves both research and development as well as collaborative testing of candidate reference materials. Once all the information is complete, monographs are posted as “For Comment.” Public comment at both stages is welcomed. HMC monographs become “Final Authorized’ through decisions of the Council of Experts. Data in support of allied reference materials is also reviewed and, if suitable, approved by the Council of Experts.
The USP Council of Experts has a specific Expert Committee—the USP Dietary Supplements and Herbal Medicines Expert Committee—to finalize HMC monographs and associated reference materials. The Expert Committee comprises volunteer scientists, academicians, and other professionals from around the world elected on the basis of their knowledge and expertise. USP has also established regional Expert Panels that provide scientific support and recommendations on HMC standards to the Expert Committee.
