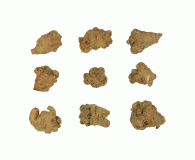Panax notoginseng Root and Rhizome
Panax notoginseng Root and Rhizome
Final Authorized Version 1.0
Panax notoginseng Root and Rhizome
DEFINITION
The article consists of the dried roots, rootlets, and rhizomes of Panax notoginseng (Burk.) F. H. Chen (Family Araliaceae) collected before flowering in autumn. It contains NLT 5.0% of total saponins calculated as the sum of notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd, and NLT 0.6% of notoginsenoside R1, on the dried basis.
SYNONYMS
Aralia quinquefolia var. notoginseng Burkill
Panax pseudoginseng var. notoginseng (Burkill) G. Hoo & C. L.Tseng
POTENTIAL CONFOUNDING MATERIALS...